The hypothalamus is a structure housed deep within the brain. It works to keep the body in a state of homeostasis, which it does by influencing the autonomic nervous system and managing hormones. A key role of the hypothalamus is appetite regulation – certain nuclei within the hypothalamus control the sensations of hunger and fullness, ultimately influencing weight gain and weight loss. Genetic disorders and damage to the hypothalamus can disrupt these crucial functions, resulting in a variety of adverse health effects including hypothalamic obesity. People with hypothalamic obesity have ravenous and insatiable appetites. In my experience, these obesities have been the most difficult to treat.
Prader-Willi syndrome, or PWS, is a genetic disorder that causes many physical, mental, and behavioral problems. PWS is characterized by poor muscle tone and failure to thrive in infancy, and subsequent obesity, learning difficulties, and short stature. PWS affects hypothalamic function – from the ages of 12 to 18 months onward, uncontrollable excessive eating results in severe obesity. A potential mediator of obesity in PWS patients is ghrelin, a hormone mainly produced in the stomach that is implicated in the regulation of hunger and stimulation of growth hormone secretion. Children with PWS develop short stature, reduced muscle mass, and increased fat mass – body composition abnormalities that resemble those seen in cases of growth hormone deficiency. Children and adolescents with PWS that I treated were most persistent and creative when it came to seeking food. A regular and representative finding in the families of these patients was a lock on the refrigerator to limit their child’s access. Parents were often exhausted by these efforts.
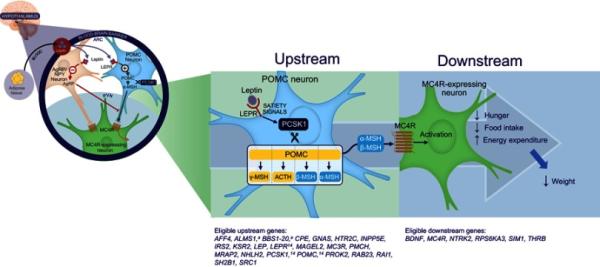
Bardet-Biedl syndrome, or BBS, is another rare disorder characterized by obesity, learning difficulties, and physical abnormalities. Multiple genetic abnormalities have been associated with BBS. The genetic abnormality in BBS is along the melanocortin-4 receptor (MC4R) pathway – which, when activated by certain neuropeptides, promotes satiety, energy expenditure, and weight loss. Mutations in this pathway can result in genetic disorders of obesity, including BBS.
Source: Eneli et al.
Brain surgery can cause hypothalamic injury and subsequent hypothalamic dysfunction. Surgery to remove craniopharyngiomas, or tumors that may grow near the hypothalamus, often damages the hypothalamus. Hypothalamic injury caused by such surgery is associated with a variety of adverse effects, including significantly greater postoperative weight gain compared to surgery patients who did not experience hypothalamic injury. The effects of weight gain from hypothalamic damage post-craniopharyngioma are immediate, uncontrollable, and heartbreaking for affected patients and families.
Recent findings provide some hope regarding the treatment of hypothalamic obesity. A study published in 2013 demonstrated efficacy of GLP-1 analogues for substantial and sustained weight loss for a small sample of adult who had suffered hypothalamic injuries. Another small study published earlier this year found that the use of Tesomet resulted in significant weight reductions compared to placebo in adult patients with hypothalamic injuries caused by surgery. The drug setmelanotide (Imcivree) targets the MC4R pathway in the hypothalamus, and has been found highly effective in the treatment of individuals with BBS-associated obesity and hyperphagia. This drug opens a whole new approach to the understanding and treatment of genetically determined obesity, and provides hope for continued innovations in this area of research.
We are grateful for the work of the Raymond A. Wood Foundation (RAWF) on hypothalamic obesity caused by brain tumors. The Foundation is a new Associate Member of the STOP Obesity Alliance. Their mission is to improve the quality of life of hypothalamic-pituitary brain survivors and is motivated to find solutions to this disabling condition.